Pneumococcal Vaccine Prevenar 13 from Pfizer Labelled in English Available in Germany
On 12 March 2020, the Paul-Ehrlich-Institut informed the public on supply problems with pneumococcal vaccines on its website.
English labelled Prevenar 13 will be commercially available, as from 28 April 2020. These doses will supplement the regular deliveries. The importation of the product was supported by a permission pursuant to Section 79 (5) German Drug Law (AMG). The vaccine doses will be distributed via the established wholesaler channels.
Details
- The imported batch is Prevenar 13, Batch No. AT5485, with expiry date in January 2022, in packages of 10 x 1 doses.
- The PZN (Pharmazentralnummer, pharmaceutical ID in Germany) is 16664469.
- Injection needles are provided in each package.
- Adhesive labels for documentation in the German vaccination document (Impfpass) are not included (manual documentation is required).
- The batch was tested by a European Official Medicines Control Laboratory (governmental laboratory, ANSM, France). It was confirmed that the manufacture conforms to the marketing authorisation and that the authorised quality criteria were adhered to.
- The readily packed products do not contain a package leaflet in German language. The secondary package (outer carton) labelled in English does not conform to anti tempering device requirements laid down in the falsified medicines directive. A serialisation of the product (i.e. equipping the package with features permitting the traceability of each package from the pharmacy right back to the manufacturer) was not feasible from the technical point of view. A verification and deactivation of the packages at the time of release by the Pharmacy are neither possible nor required. The scanning of the code on the package by the pharmacist would result in an error message in securePharm.
- The German package leaflet and the summary of product characteristics are available for download on this page.
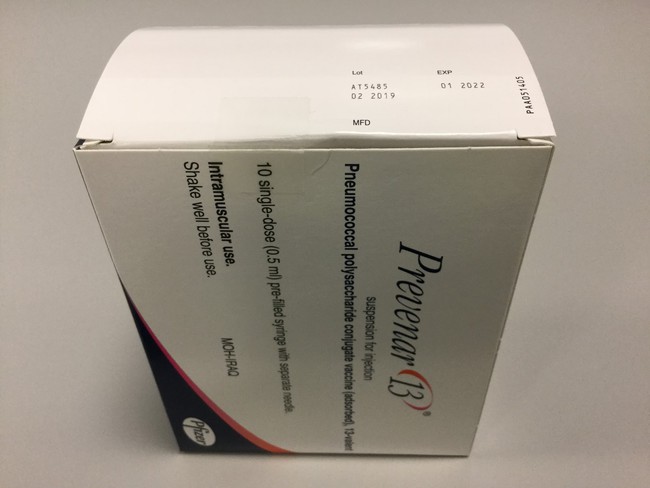 Packaged product (10 x 1 dose)
Source: Pfizer Limited, UK
Packaged product (10 x 1 dose)
Source: Pfizer Limited, UK
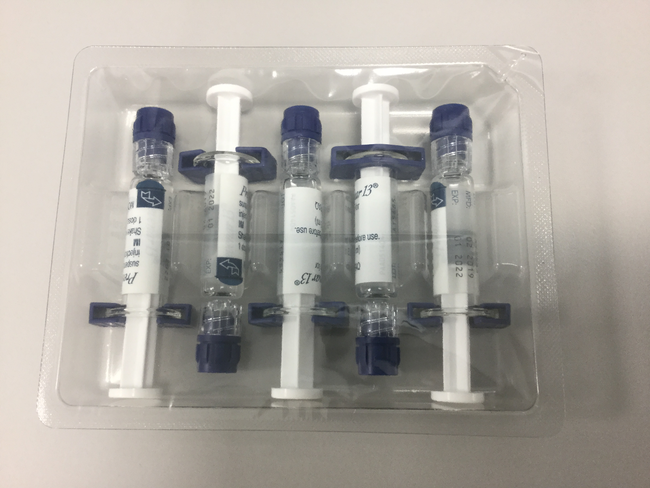 Blistered syringes 5x1 dose, packaging contains 2 blister packs with 5 syringes each
Source: Pfizer Limited, UK
Blistered syringes 5x1 dose, packaging contains 2 blister packs with 5 syringes each
Source: Pfizer Limited, UK
How Should the Vaccine be Used?
Special advice given by the German Advisory Committee for Immunisation Practices (Ständige Impfkommission, STIKO) during the period of shortage should be followed.
Background
The Coronavirus-2 pandemic has led to an increased demand for pneumococcal vaccines resulting in the present supply shortages. In its role as a senior federal authority, the Paul-Ehrlich-Institut, Federal Institute for Vaccines and Biomedicines, is responsible for the quality, safety, and efficacy and the official batch release testing of vaccines. In addition, it is committed to ensuring the availability of these medicines. The application of special rules laid down in the German Medicines Act, in this case, makes it possible that, if a supply shortage is identified by the Federal Ministry of Health (BMG), an exception can be made for imports if, based on Section 79 (5) AMG, a permission by the federal state (Bundesland) authorities has been pronounced. In this case English labelled products can be imported to Germany.
This website contains an overview of all current supply shortages of vaccines for human use in Germany, which is regularly updated.
Our email newsletter “Safety and availability – information on medicines safety, notes for action in the event of delivery shortages of vaccines for human use" contains information on current availabilities and delivery shortages on a regular basis.




