SafeVac 2.0 App – Information on Booster Vaccination now Possible
With the smartphone app SafeVac 2.0 developed by the Paul-Ehrlich-Institut, vaccinated people can report digitally on how they tolerated their vaccination against COVID-19. Until now, only information on the first and second vaccination could be reported in the app. Due to the booster vaccination recommended by the Standing Commission on Vaccination (Ständige Impfkommission, STIKO) and the great interest of the population, the app has been updated. With the update 2.3.1, participants aged 12 and older can now also enter in the app how well they tolerated their booster vaccination against COVID-19. The booster vaccination information can also be entered retrospectively.
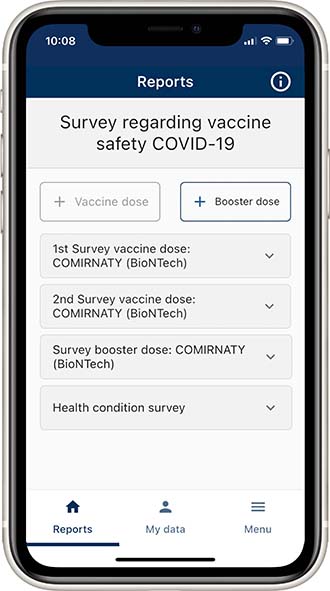
With the current app version, it is possible to enter booster vaccination information. SafeVac app users can enter a booster dose via the newly added "Booster dose" button provided that they are already registered with the SafeVac app and have participated in the survey with the first vaccinations. This entered dose will also be displayed in a single block "Survey booster dose".
 Source: Paul-Ehrlich-Institut
Source: Paul-Ehrlich-Institut
The information on the booster vaccination is also possible retrospectively (provided that the registration in the SafeVac app took place at the time of the basic immunisation): the 48-hours rule as for the survey on the first vaccination does not apply here.
The assessment of the reports from the last few months has shown that most side effects occur up to seven days after the vaccination date. Therefore, the survey on booster vaccinations only asks for a single reporting date.
Participants also have the opportunity to report additional adverse events in the SafeVac app within the six-month and 12-month health status surveys. In addition, reporting of potential adverse effects is also possible at any time and for everyone via the website www.nebenwirkungen.bund.de.



