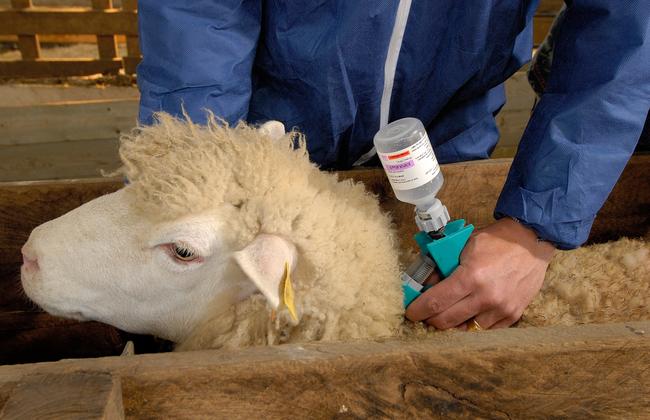
Protection Against Bluetongue – Exemption for Vaccines Extended
In June 2024 the Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft, BMEL) authorised the use of the three BTV-3 vaccines for the protection of sheep and cattle that had been designated by the Paul-Ehrlich-Institut. The authorisation of BULTAVO 3, BLUEVAC-3, and Syvazul BTV 3 was issued for a period of six months and was set to expire on 6 December 2024. In a regulation that came into force on 28 November 2024, the BMEL lifted the initial time limit and extended the use of these three vaccines until a vaccine is approved for marketing authorisation. This means that livestock owners in Germany can continue to vaccinate their cattle and sheep against bluetongue.
 Source: Leitenberger Photography/Shutterstock.com
Source: Leitenberger Photography/Shutterstock.com
Administration of the permitted BTV-3 vaccines has so far shown that timely and proper primary vaccination of susceptible animals with the BTV-3 vaccines protects these animal populations from the harmful effects of BTV-3 infection on animal health, such as serious illness or death. Vaccination helps to prevent economic damages associated with BTV-3 outbreaks, which business owners experience in the form of animal losses, drops and/or losses in production, and costs for veterinary treatments.
The following three vaccines can be used for the prevention of bluetongue:
- BULTAVO 3 – marketed by Boehringer Ingelheim Vetmedica GmbH, Germany
- BLUEVAC-3 – marketed by CZ Vaccines S.A.U., Spain/CEVA Tiergesundheit GmbH, Germany
- Syvazul BTV 3 – marketed by Laboratorios Syva S.A., Spain/Virbac Tierarzneimittel GmbH, Germany
Reporting Adverse Events after Vaccine Use
The Paul-Ehrlich-Institut keeps a centralised record of reports of adverse events (AEs) after the use of vaccines in veterinary medicine and evaluates the reports (pharmacovigilance). This also applies to reports submitted in connection with vaccinations against BTV-3. The goal of the evaluations is to analyse the benefit-risk ratio of the non-authorised BTV-3 vaccines when used properly, while also considering the urgent situation and the limited availability of data currently.
Reports of suspected adverse events after the use of BTV-3 vaccines can, as with all veterinary vaccines, be submitted to the Paul-Ehrlich-Institut in a structured manner via the online reporting portal or communicated directly by email.
Contact
Email: vetmittelsicherheit@pei.de
top



