Batch Testing (Veterinary)
Immunological veterinary medicinal products (sera, vaccines, immunomodulators, tuberculins, and other veterinary medicinal products) are subject to official batch testing in accordance with the Animal Vaccine Regulation and the Veterinary Medicinal Products Act. Each batch (production unit) must be released by the Paul-Ehrlich-Institut (PEI) before being placed on the market in Germany.
The requirements for the proof of product quality, in particular the quality of immunological veterinary medicinal products, are regulated in Regulation (EU) 2019/6. The regulation distinguishes between a protocol review only and additional experimental testing. The review or testing used for an immunological veterinary medicinal product is regulated in the general guideline titled "EU Administrative Procedure for Official Batch Release of Immunological Veterinary Medicinal Products in Application of Article 128 of Regulation (EU) 2019/6", which regulates official batch release based on testing by a control laboratory.
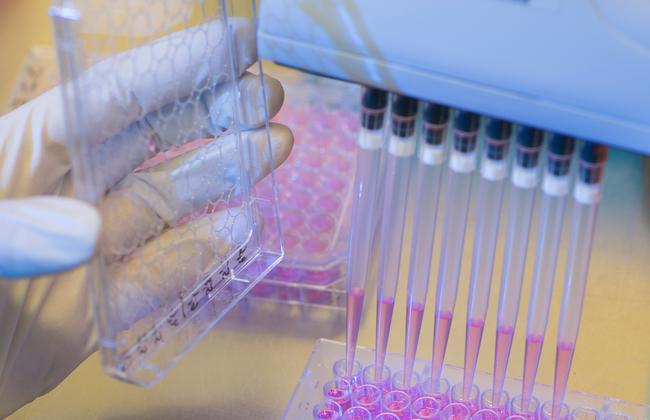
The Friedrich-Loeffler-Institut (FLI) is responsible for the marketing authorisation and batch testing of products not designed for use in animals (in vitro diagnostic medical devices, IVDs).
Upon request, the Paul-Ehrlich-Institut checks the manufacturing documents that are submitted and the results of the quality controls and, if necessary, examines the test samples. If the batch meets the quality, efficacy and safety criteria laid down in the marketing authorisation of the veterinary medicinal product, the Paul-Ehrlich-Institut will issue a national decision on the release of the batch. This approval notice allows the pharmaceutical company to market the batch in Germany.
The Paul-Ehrlich-Institut also examines immunological veterinary medicinal products intended for marketing outside Germany and issues test reports on them.
Batch testing is carried out in accordance with the procedures and regulations of the Official Medicines Control Laboratories (OMCLs) of the Member States of the European Union (EU), the European Economic Area (EEA), and Switzerland. The OMCLs have joined forces in a network coordinated by the European Directorate for the Quality of Medicines and HealthCare (EDQM). The Paul-Ehrlich-Institut is represented in the OMCL network as a state control laboratory.
Guide – Submitting an Application for Batch Release
The following diagram shows the application process for batch release:
Flow Chart: Guide to Submitting an Application for Batch Release
top



