Procedures and Timelines
Submission of Clinical Trial Applications
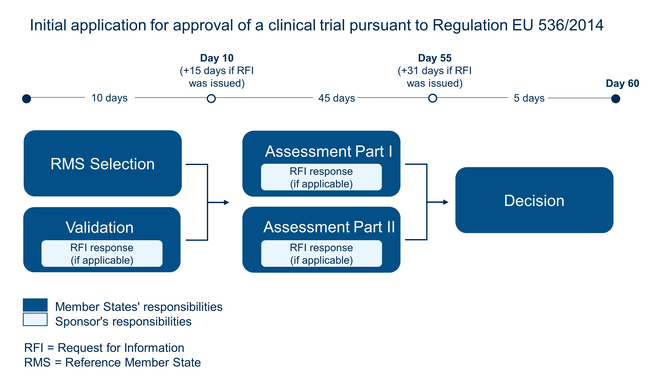
The conduct of a clinical trial in the European Union (EU) is subject to approval pursuant to the Clinical Trials Regulation (EU) No. 536/2014. The regulation divides the approval procedure for clinical trials into the following sections:
- Application submission
The sponsor submits the application for approval of the clinical trial in the common European Clinical Trials Information System (CTIS) portal. - Validation
The Reference Member States (RMS) named in the application examine the application for completeness and validity. The Paul-Ehrlich-Institut (PEI) carries out this task for vaccines and biomedicines. - Assessment
The higher federal authority and the respective ethics committee evaluate the application and issue an opinion. If the documents submitted are insufficient or errors are identified, these issues will be communicated to the sponsor (Request for Information, RFI). The sponsor then has the opportunity to fix the errors. - Decision on approval
The higher federal authority decides with the other participating Member States on the clinical trial approval.
Approval Process and Processing Times

Shorter Processing Times for Mono-National Clinical Trials
The Medical Research Act (Medizinforschungsgesetz, MFG) established a shorter processing time for mono-national clinical trials within section 40 subsection 4 of the AMG. The shorter processing time means that initial applications for mono-national clinical trials will be assessed within 26 days of successful validation by the responsible higher federal authority and the responsible ethics committee. If the application has no errors, a decision can be made within 31 days of validation.
The ethics committees and the two higher federal authorities, the Paul-Ehrlich-Institut and the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM), also guarantee shortened deadlines for the processing of mono-national substantial modification notifications. This guarantee means that a decision on a defect-free application will be issued 24 days after successful validation at the latest.
top



