Processing Statistics
This section shows the processing statistics on clinical trials for which the Paul-Ehrlich-Institut is the competent authority.
Overview of application figures from 1995 - 2022
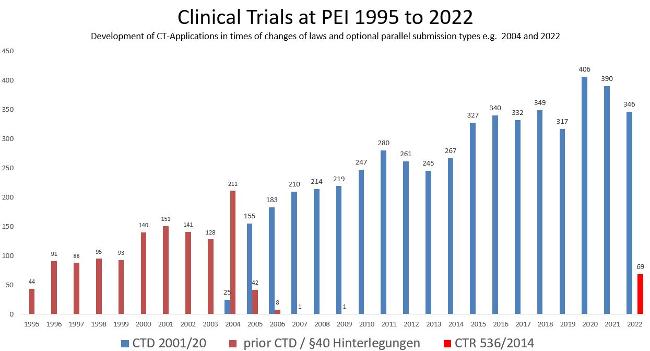

The figure shows the general development of applications for approval of clinical trials at the Paul-Ehrlich-Institut and the transition periods of parallel submissions from 2004 to 2009 and in 2022. The number increased from 44 in 1995 to 416 in 2022 .
Prior to August 2004, submissions for clinical trials were only stored at the Paul-Ehrlich-Institut.
From August 2004 to 31 January 2023, applications were possible under Directive 2001/20/EC as implemented in the German Medical Drugs Act (AMG) and GCP-V for all investigational medicinal products, i.e., submission in CTD format.
From 31 January 2022, applications according to Regulation 536/2014/EC will be possible for medicinal products covered by Regulation 2001/83/EC, i.e., submission in CTR format.
For medicinal products not covered by Regulation 2001/83/EC the provisions of the AMG/ Ordinance on GCP (GCP-V) will continue to be applied in Germany.
Initital clinical trial applications 2022
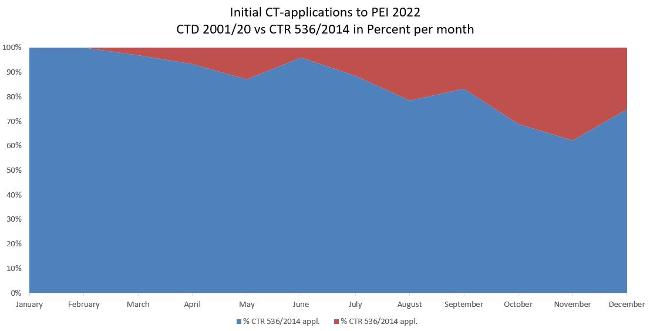

The figure shows the application numbers of clinical trials in 2022 and the distribution in percent depending on the application according to Directive 2001/20/EC as implemented in the German Medical Drugs Act (AMG) and Ordinance on GCP (GCP-V) or Regulation 536/2014/EC. Adoption of application according to Regulation 536/2014/EC was hesitant.
Absolute numbers of application 2022
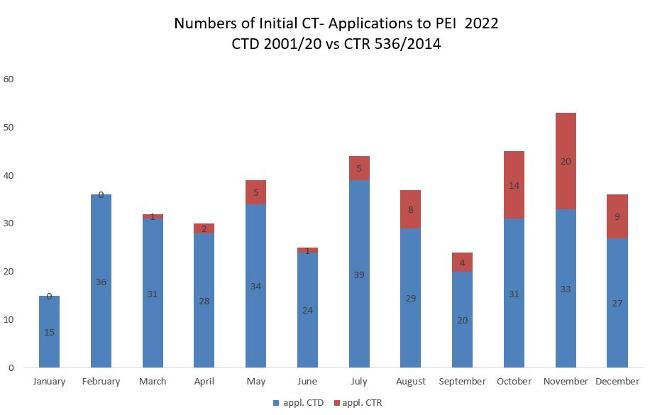

The figure shows the absolute numbers of application according to Directive 2001/20/EC in the implementation in the German Medical Drugs Act (AMG) and Ordinance on GCP (GCP-V) or Regulation 536/2014/EC.
Overview of all requests
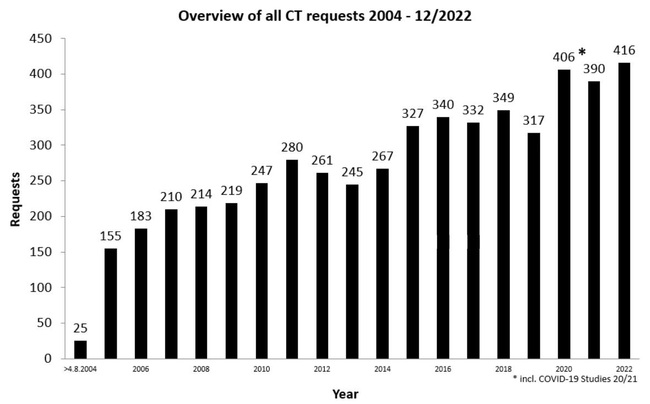

Distribution of Clinical Trial Authorisations by commercial and non-commercial sponsors
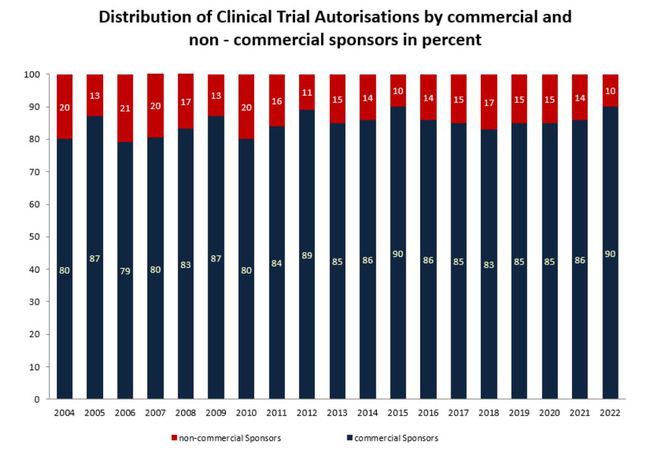

* without clinical trial submitted before the implementation of Directive 2001/20/EC
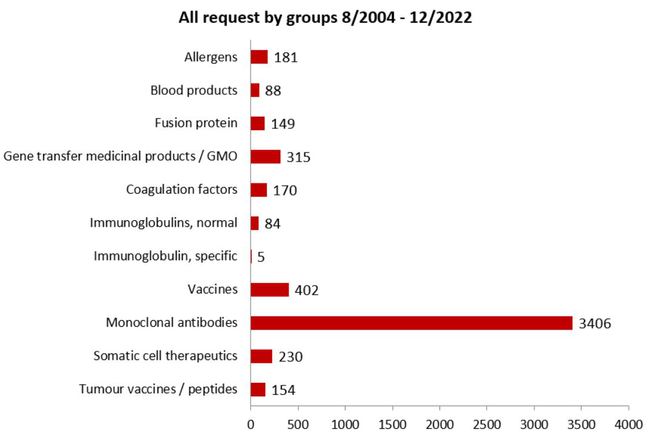
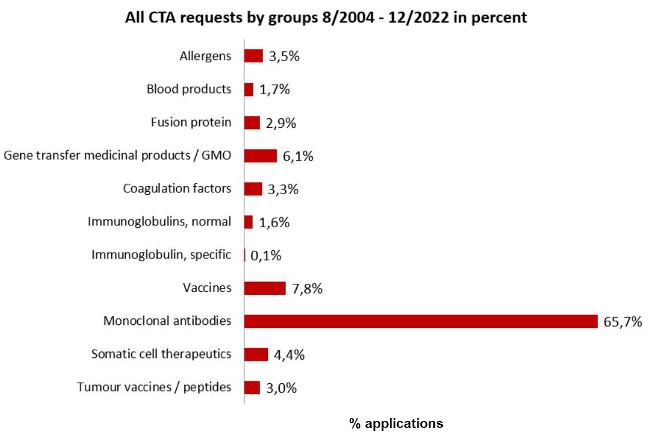
All requests by product groups
Decisions on clinical trial applications
(August 2004 - 2022)
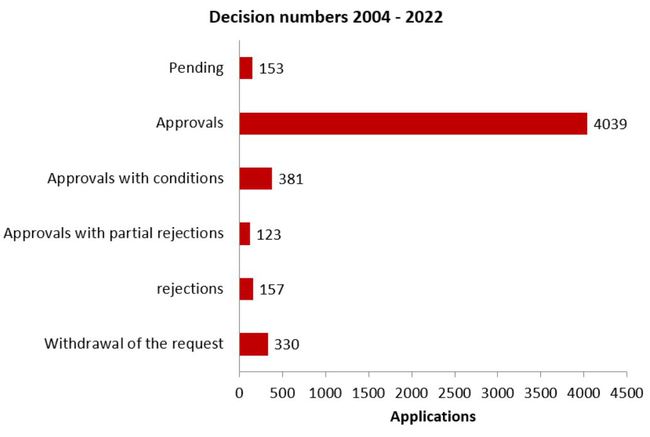
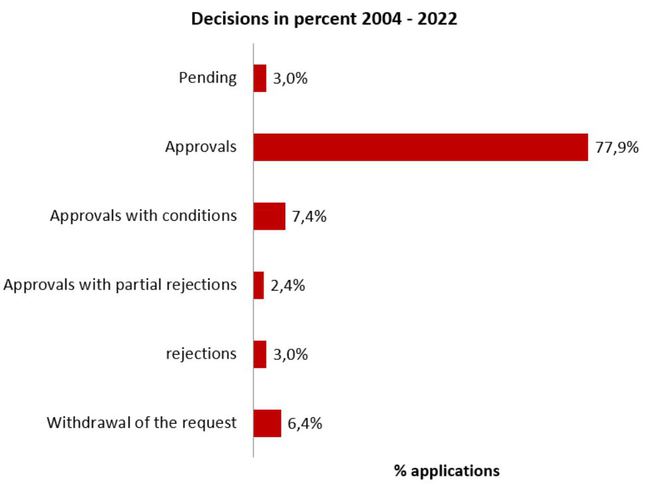
Protest procedures may alter the outcome of the procedure in retrospect compared with the data of the previous year.
Amendments to clinical trial authorisations
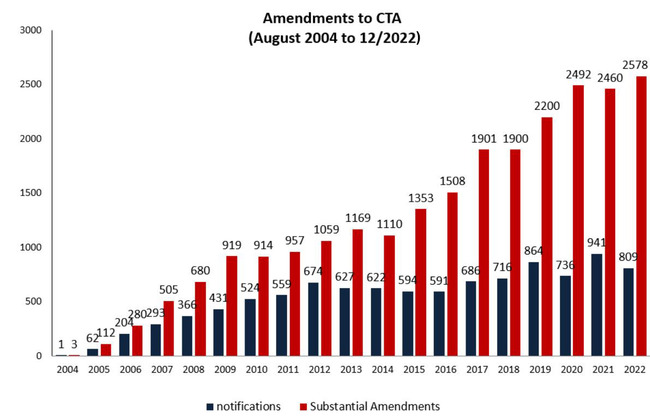

| Type of request | Portion | Type of notification |
|---|
| Notifications | 100% | favourable |
Subst. Amendments (requiring authorisation)
Distribution of decisions | 0,20% | pending |
| 96,50% | approvals |
| 0,50% | approval with conditions |
| 1,40% | partial Rejection |
| 0,45% | rejections |
| 0,90% | withdrawal of Amendment |
The table is representing the figures for amendments to clinical trial requests requiring an authorisation and amendments for notification only from 2004 to 12/2022.
Submission of Annual safety reports to the PEI
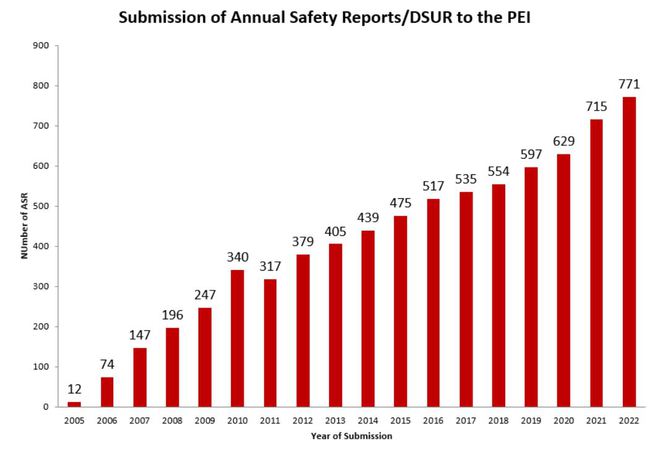

An Annual Safety Report has to be submitted to the Paul-Ehrlich-Institut for clinical trials, that are performed in Germany for longer than one Year.
top













