Research in the Immunology Division
 Source: Paul-Ehrlich-Institut
Source: Paul-Ehrlich-Institut
The Immunology Division is responsible for the authorisation and batch release of immunotherapeutics such as antibodies, immunoglobulins, and antisera. The research projects in the Research Immunology Section contribute outstanding scientific expertise to ensure high-quality analyses of novel biomedical strategies and innovative experimental approaches in various areas of immunology.
Research Focus
One research focus is the investigation of the immunomodulating effect of adjuvants or novel excipients, such as lipid nanoparticles (LNPs), on human immune cells. Adjuvants are added to vaccines in which the antigen alone triggers only a moderate or inadequate immune response. LNPs are used to stabilise and facilitate the uptake of medicinal substances and mRNA vaccines. The results of this research contribute to a better understanding of the effect of adjuvants and LNPs and support us in evaluating new vaccines during the authorisation process.
We also analyse strategies used by pathogens to bypass the immune defence and prevent their detection by the immune system. We are working with the obligate intracellular Leishmania parasites, the pathogen that causes the tropical disease leishmaniasis, and primary lymphocytes derived from human blood as a model of an intracellular pathogen that infects human immune cells.
Another research topic concerns the study of serious side effects of biomedicines such as monoclonal antibodies or blood coagulation products. We analyse the underlying molecular and cellular immunological mechanisms of these adverse effects and examine both innate and adaptive immune responses. We seek to develop meaningful in vitro systems that are able to reliably map known serious adverse events of these medicinal products. These include the formation of anti-drug antibodies (ADA) or vascular leakage syndrome (VLS), i.e. the pathological permeability of vessels.
In a further approach, we explore the biological mechanisms that lead to the formation of a diverse T-cell repertoire and its long-term preservation in the organism (homeostasis). A highly diverse T-cell repertoire is essential for the immune system to be able to react against new or changed pathogens. To understand the underlying mechanisms, comparisons are made between T-cell populations that are sourced from different organs, originate from different individuals, or are isolated after experimental manipulations. These comparisons allow conclusions to be drawn about the mechanisms that influence the T-cell repertoire and decide whether and what type of immune response can be triggered. We are also interested in learning how T cells of different specificity compete with each other or how this is controlled in the immune system in such a way that a broad T-cell repertoire is maintained – often, but unfortunately not always, into old age.
In addition, we study the immunomodulatory effects of endogenous nitro fatty acids as novel inflammatory mediators. Nitro fatty acids (NFAs) are endogenously formed and highly potent lipid-based anti-inflammatory signalling molecules for which pronounced anti-inflammatory and cell-protective effects have already been demonstrated in a variety of animal studies. NFAs can arise during inflammatory processes in the human body as well as be exogenously absorbed with food. When the body forms its own NFAs, unsaturated fatty acids such as oleic acid or conjugated linolenic acid are nitrated by reactive nitrogen species (e.g. NO, NO2) in a non-enzymatic reaction. This results in the formation of a reactive and electrophilic nitroolefin unit, what is known as a Michael acceptor (MA). Via this MA group, NFAs can react covalently with nucleophilic side chains of amino acids, such as cysteines or histidines, in a reversible nitroalkylation process and thus influence protein functions. Not only can these studies provide fundamental insights into inflammatory processes of the immune system, but they can also create the basis for novel immunomodulatory and anti-inflammatory therapies.
Leukocytes (granulocytes, monocytes, and lymphocytes) can only fulfil their task of detecting foreign antigens and eliminating pathogens if they emigrate from the intravascular space to extravascular infected tissue. To do so they must adhere to the vascular wall and actively overcome the endothelial barrier. This results in various interactions between leukocytes and the vascular endothelium involving a number of mediators (interleukins, chemotactic factors, growth factors, etc.). In another project, we are investigating leukocyte-endothelial interactions and their pharmacological influence on the basis of model substances in order to create the basis for new (biomedicinal) therapeutics that act by attacking leukocyte-endothelial interactions.
 Research focal points of the Research Immunology Section.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research focal points of the Research Immunology Section.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Head of Division
Professor Dr Ger van Zandbergen
Publications
Email: Ger.vanZandbergen@pei.de
Research Group Head
Professor Dr Thorsten Jürgen Maier
Publications
Email: ThorstenJuergen.Maier@pei.de
Deputy Research Group Head
Professor Dr Zoe Waibler
Publications
Email: Zoe.Waibler@pei.de
Research Projects
Immunomodulating Effects of Adjuvants and LNPs
- Research on the immunomodulating effect of adjuvants on primary human cells of the innate and adaptive immune systems,
- Gaining insights into the individual immune signatures of the adjuvants to better understand their function and to support the evaluation of new vaccines during the authorisation process.
 We investigate the effect of adjuvants and LNPs on the activation of innate immune system cells (e.g. dendritic cells, granulocytes, and macrophages) and on the acquired immune system (e.g. T, B, and NK cells), both of which are important for the immune response. Different adjuvants stimulate immune cells in specific ways and with varying strengths.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
We investigate the effect of adjuvants and LNPs on the activation of innate immune system cells (e.g. dendritic cells, granulocytes, and macrophages) and on the acquired immune system (e.g. T, B, and NK cells), both of which are important for the immune response. Different adjuvants stimulate immune cells in specific ways and with varying strengths.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research Project Head
Professor Dr Ger van Zandbergen
Publications
Email: Ger.vanZandbergen@pei.de
Serious Side Effects of Biomedicines or Immunological Medicinal Products
Role of innate and adaptive immune cells in the formation of ADA and in the immunogenicity of coagulation factor VIII products and importance of immunological danger signals.
 It is not yet clear why some patients treated with FVIII develop ADA, while others do not.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
It is not yet clear why some patients treated with FVIII develop ADA, while others do not.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
The EU consortium imSAVAR (Immune Safety Avatar; nonclinical mimicking of the immune system effects of immunomodulatory therapies) has set itself the goal of developing new concepts for testing immunomodulatory therapies. The focus is on the improvement of existing model systems and the development of new ones. One potential result could be the improved capability to map and thus predict serious adverse events of biomedicines. As a partner in this consortium, we seek to address the following issues:
- How do immune cells contribute to the formation of VL?
- How do immune cells and endothelial cells interact?
- How can we mirror VL in vitro?
- How could an in vitro assay system predict VL induced by biomedicines?
 A number of (immunological) factors, including treatment with biomedicines, can lead to vascular leakage, a serious and life-threatening permeability of vessels.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
A number of (immunological) factors, including treatment with biomedicines, can lead to vascular leakage, a serious and life-threatening permeability of vessels.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research Project Head
Professor Dr Zoe Waibler
Publications
Email: Zoe.Waibler@pei.de
Strategies of Leishmania for Circumvention of the Immune Defence
- Identification of parasite genes or proteins, which play a role in the survival or replication of these parasites in human host cells, represent potential target structures for medicines or can be used for the development of a vaccine against leishmania.
- Infection experiments conducted using primary human host cells of the innate immune system, in particular with macrophages.
- Research into the effects of infection with Leishmania including those on the acquired immune system, especially on T cells.
- Study of Leishmania transfer and spread to new host cells.
We also use this human leishmaniasis infection model to examine the functionality of various authorised immunomodulatory antibodies, such as anti-tumour necrosis factor alpha or checkpoint inhibitors like programmed cell death protein 1 (PD-1) inhibitors, which intervene in inflammatory processes or immune activation.
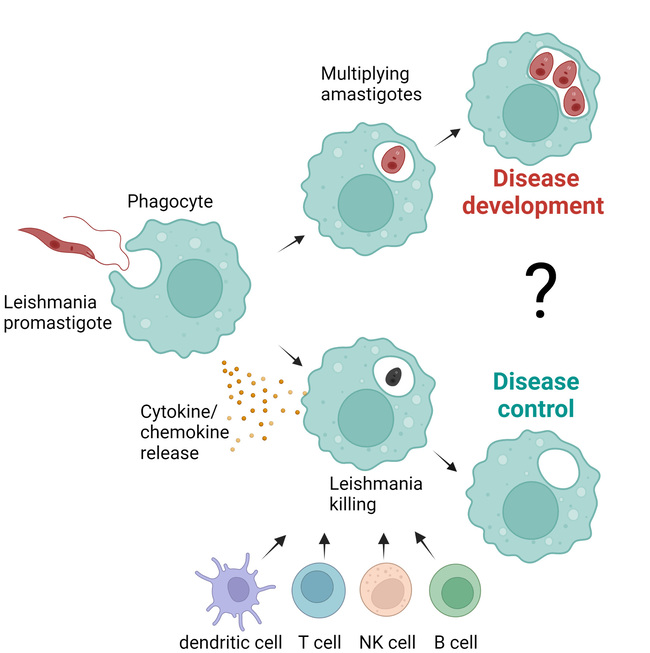 After Leishmania promastigotes are absorbed into phagocytes, the parasites transform into the replicative amastigote form, which leads to the development of the disease. However, infected phagocytes can also release pro-inflammatory cytokines and chemokines, which can activate other immune cells (e.g. dendritic cells, T, B and NK cells) and bring the infection under control.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
After Leishmania promastigotes are absorbed into phagocytes, the parasites transform into the replicative amastigote form, which leads to the development of the disease. However, infected phagocytes can also release pro-inflammatory cytokines and chemokines, which can activate other immune cells (e.g. dendritic cells, T, B and NK cells) and bring the infection under control.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research Project Head
Professor Dr Ger van Zandbergen
Publications
Email: Ger.vanZandbergen@pei.de
Immunological Fundamentals/T-Cell Development and Homeostasis
- Detection of the T-cell repertoire of T-cell populations included in the research, such as helper, killer or regulatory T-cells, using next-generation sequencing (NGS),
- Comparisons between T-cell populations sourced from different lymphatic organs/tissues, originating from different individuals, or are isolated after different experimental modifications,
- Bioinformatic analysis is carried out in collaboration with specialists who have developed mathematical approaches to modelling the immune system.
Conclusions can be drawn from these comparative studies as to how the immune system, despite the presence of competing lymphocytes, can maintain a broad T-cell repertoire or how an immune reaction determines how the immune response proceeds. In addition, special techniques are used in this research (including gnotobiotics) to understand the influence of germs, e.g. the natural intestinal flora (microbiome), on these processes.
Investigations on the Immunomodulatory Effects of Endogenous Nitro Fatty Acids and Structurally Related Lipid Mediators
- Identification of the direct cellular targets of NFAs by means of cellular enzyme activity and protein expression studies as well as proteomics methods,
- Study of the immunomodulatory effect of NFAs in cellular and animal models of inflammatory responses and tumour development,
- Studies on the structure-activity relationship of the immunomodulatory effect of NFAs.
The aim of our research is to better understand the predominantly immunomodulatory as well as the anti-tumorigenic effects of these endogenous mediators. Not only can this research provide fundamental insights into inflammatory processes of the immune system, it can also create the basis for novel immunomodulatory and anti-inflammatory therapies.
 Formation of nitro fatty acids and representation of their biological effects.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Formation of nitro fatty acids and representation of their biological effects.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research Project Head
Professor Dr Thorsten Jürgen Maier
Publications
Email: ThorstenJuergen.Maier@pei.de
Studies of Leukocyte-Endothelial Interactions and Their Pharmacological Influence
- Preliminary experiments showed that the sepsis therapeutic imipenem caused a pronounced and significant inhibition of the adhesion of primary human monocytes to human endothelial cells.
- Fundamental findings on the pharmacological influence of leukocyte-endothelial interaction and leukocyte migration are to be achieved using imipenem as a model substance. These findings will lay the foundation for new (biomedical) therapeutics that act by attacking leukocyte-endothelial interactions.
 Studies of the effect of the model substance imipenem on leukocyte-endothelial interactions.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Studies of the effect of the model substance imipenem on leukocyte-endothelial interactions.
Source: Paul-Ehrlich-Institut (created with BioRender.com)
Research Project Head
Professor Dr Thorsten Jürgen Maier
Publications
Email: ThorstenJuergen.Maier@pei.de
top










